Organic Chemistry Lesson of the Day – The 4 Conformational Isomers of Butane
June 18, 2015 Leave a comment
In a previous Chemistry Lesson of the Day, I introduced the simplest case of conformational isomerism – the staggered and eclipsed conformations of ethane. The next most complicated case of conformational isomerism belongs to butane. Here are the Newman’s projections of the 4 possibilities.
Modified image courtesy of Avitek from Wikimedia.
The conformational isomers are named with respect to the proximity of the 2 methyl groups. The dihedral angle between the 2 methyl groups, θ, is below each Newman projection. From left to right, the conformational isomers are:
- fully eclipsed (θ = 0 degrees)
- gauche (θ = 60 degrees)
- eclipsed (θ = 120 degrees)
- anti (θ = 180 degrees)
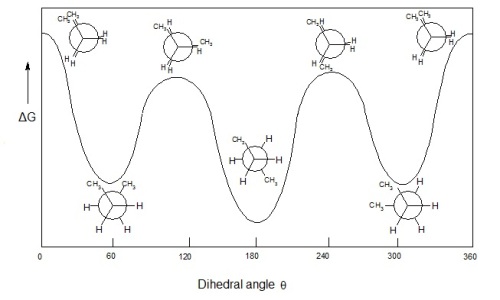
Clearly, the fully eclipsed conformation has the most steric strain* between the 2 methyl groups, so its internal energy is highest.
Clearly, the anti conformation has the lowest steric strain between the 2 methyl groups, so its internal energy is lowest.
The gauche conformation has less steric strain than the eclipsed conformation, so its internal energy is the lower of the two conformations.
From lowest to highest internal energy, here is the ranking of the conformation isomers:
- anti
- gauche
- eclipsed
- fully eclipsed
This can be visualized by the following energy diagram.
Image courtesy of Mr.Holmium from Wikimedia.
*As mentioned in my previous Chemistry Lesson of the Day on the 2 conformational isomers of ethane, there is some controversy about what really causes the internal energy to increase in eclipsed conformations. Some chemists suggest that hyperconjugation is responsible.
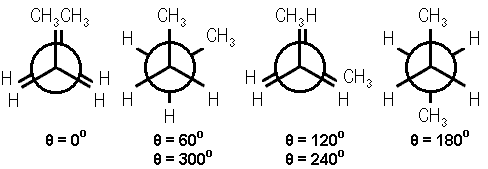




Recent Comments