Detecting Unfair Dice in Casinos with Bayes’ Theorem
October 30, 2013 1 Comment
Introduction
I saw an interesting problem that requires Bayes’ Theorem and some simple R programming while reading a bioinformatics textbook. I will discuss the math behind solving this problem in detail, and I will illustrate some very useful plotting functions to generate a plot from R that visualizes the solution effectively.
The Problem
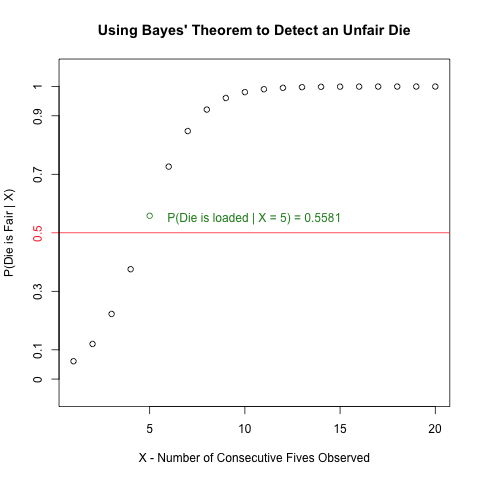
The following question is a slightly modified version of Exercise #1.2 on Page 8 in “Biological Sequence Analysis” by Durbin, Eddy, Krogh and Mitchison.
An occasionally dishonest casino uses 2 types of dice. Of its dice, 97% are fair but 3% are unfair, and a “five” comes up 35% of the time for these unfair dice. If you pick a die randomly and roll it, how many “fives” in a row would you need to see before it was most likely that you had picked an unfair die?”
Read more to learn how to create the following plot and how it invokes Bayes’ Theorem to solve the above problem!
Recent Comments